This web page was produced as an assignment for Gen677 at UW-Madison Spring 2010
Domains
Domains for the ABL1 protein were found using the program SMART (1) and Pfam (2). Both programs indicated there are four domains in the protein.
SH3 domain
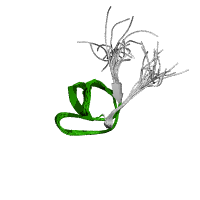
SH3 domain structure (2)
Src homology 3 (SH3) domain are known to bind to target protein through sequences containing proline and hydrophobic amino acids. This allows it to play a significant role in cell signaling. Even though the function is not completely understood, possible functions that have been suggested include increasing local concentration of proteins, altering subcellular location, and mediation the assembly of large multiprotein complexes. (1, 2)
It is approximately 50 amino acids in length which form into five to six beta strands which arrange into two tightly packed anti-parallel beta sheets. The proline and hydrophobic amino acids provide a low affinity binding site for ligands which is strengthened through multiple interactions with the ligand.
SH2 domain

SH2 domain structure (2)
Src homology 2 (SH2) also plays a role in cell signaling. It binds to phosphotyrosine-containing polypeptides with more specificity formed via interactions with residues other than phosphotyrosine. The function of the SH2 domain is supported by more research than the SH3 domain and is to be a key regulatory module of intracellular signaling. This is possible by phosphotyrosine-containing target peptides having a higher affinity to sequence-specific SH2 domains which recognize three to six amino acids C-terminal to the phosphorylated tyrosine. (1, 2)
The SH2 domain is approximately 100 amino acids in length and comprises of a central, hydrophobic anti-parallel beta sheet and two alpha-helices.
Protein Tyrosine Kinase
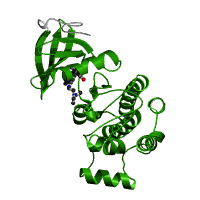
Protein tyrosine kinase domain structure (2)
The protein tyrosine kinase is a tyrosine-specific phosphotransferase which plays a role in multiple cellular processes including division, proliferation, apoptosis, and differentiation. Kinases specifically are a group of enzymes that have a catalytic subunit which transfers phosphate groups from nucleotide triphosphates such as ATP to another protein's side chain, changing the conformation and the function of that protein. Some of the changes in function may include a change in enzyme activity, cellular location, or association with other proteins. Due to its cellular roles, the catalytic subunits in the protein tyrosine kinase domain are highly conserved and, as a result, large screens are performed for kinase-inhibitors that could be used as treatments for specific diseases. (1, 2)
F-Actin Binding Domain
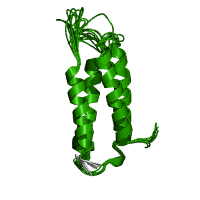
F actin binding domain structure (2)
The F-actin binding domain (FABD) is composed of four anti-parallel alpha-helices that binds to F-actin in the cell. This may result in cytoplasmic retention, subcellular distribution of the protein, and/or inhibition of the protein.

